

Ignore groups three through 12 for this video. So one, two, three, four,įive, six, seven, and eight.
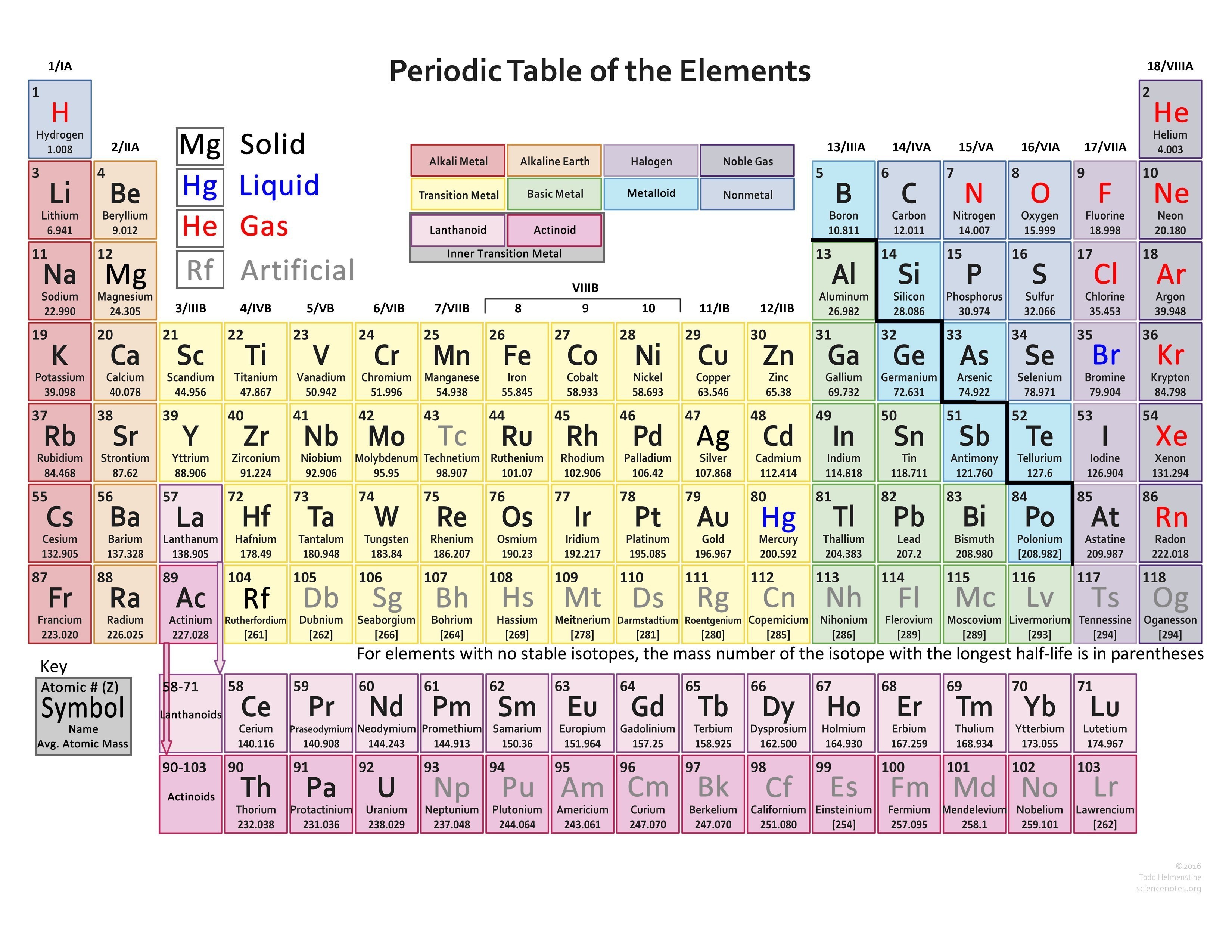
Through eight system for classifying groups. Valence electrons for elements in the main groups.
PERIODIC TABLE OF ELEMENTS WITH VALENCE ELECTRONS HOW TO
Periodic table, let's see how to determine the But the octet rule is not always followed.Ĭlassified our elements into groups on the NOTE: The Pauli exclusion principle is one of the few laws that all of the elements always obey. You have to take all of the factors into consideration to predict how an element might behave. So, with chemistry, there are many competing factors that affect how elements behave, so you cannot just take a single rule and insist or suspect that every element must necessarily follow that rule. So, while the octet rule is a convenient rule of thumb, the reality of chemistry is much, much more complicated. There are many considerations as to what does or does not make an atom with a particular electron configuration reactive or nonreactive. There is no p subshell in shell number 1, so that is part of why the lightest elements do not follow the octet rule (there are other reasons). The octet rule follows from the energetic favorability of having both the s and p subshells either completely empty or completely full. Note: H₂ is stable, but it is quite reactive. Hydrogen does not follow the octet rule, so that is part of why H₂ is stable. So, the octet rule is not a strict law of science that is always followed. Other elements, such as S and P, sometimes do not follow the octet rule. Boron usually does not follow the octet rule, though it sometimes does. H, He, Li, and Be never follow the Octet Rule. It is placed there because it is practically inert, like the other noble gases. We just place it there because it is unique and does not really fit into any group. 1995 - 2023.Helium is not truly a member of Group 18 (or Group VIII in the older system). Periodic Table of Elements - Magnesium - Mg. If you need to cite this page, you can copy this text: This database focuses on the most common chemical compounds used in the home and industry. Molar mass calculations are explained and there is a JavaScript calculator to aid calculations.

Molar Mass Calculations and Javascript Calculator.Introduces stoichiometry and explains the differences between molarity, molality and normality. Related ResourcesĪnswers many questions regarding the structure of atoms. Common Chemical Compounds of Magnesium ReferencesĪ list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.Additional Notes: In 1755 Joseph Black in Edinburgh Scotland recognized magnesium as an element, however, it wasn't issolated until 1808 by Sir Humphrey Davy.Also used in fireplace bricks, flashbulbs, pigments and filters. Uses of Magnesium: Used in alloys to make airplanes, missiles, racing bikes and other things that need light metals.Primary mining areas are Austria, China, Poland, Russia, USA, India, Greece and Canada. World production is around 350,000 tons per year. Sources of Magnesium: Usually obtained by electrolysis of melted magnesium chloride (MgCl 2) found in sea water.Name Origin: Greek: From Magnesia a district of Thessaly.Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. Vapor Pressure = C Regulatory / Health.Enthalpy of Vaporization: 128.7 kJ/mole.Enthalpy of Atomization: 148.5 kJ/mole 25☌.Reacts with hot water and burns in air when ignited. Conductivity Electrical: 0.226 10 6/cm Ω.Coefficient of lineal thermal expansion/K -1: 26.1E -6.Valence Electron Potential (-eV): 40 Physical Properties of Magnesium.Electronegativity: 1.31 (Pauling) 1.23 (Allrod Rochow).Electrochemical Equivalent: 0.45341g/amp-hr.Valence Electrons: 3s 2 Electron Dot Model.Number of Neutrons (most common/stable nuclide): 12.Number of Electrons (with no charge): 12.Electrons per Energy Level: 2,8,2 Shell Model.Electron Configuration: 1s 2 2s 2p 6 3s 2.Cross Section (Thermal Neutron Capture)σ a/ barns : 0.063.Swedish: Magnesium Atomic Structure of Magnesium.Series: Alkali Earth Metals Magnesium's Name in Other Languages.Common Chemical Compounds of Magnesium Overview of Magnesium.In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies. Common chemical compounds are also provided for many elements. Skip to site menu on this page Periodic Table of Elements Element Magnesium - MgĬomprehensive data on the chemical element Magnesium is provided on this page including scores of properties, element names in many languages, most known nuclides of Magnesium.


 0 kommentar(er)
0 kommentar(er)
